- Research Article
- Open access
- Published:
Determination of Three-Dimensional Left Ventricle Motion to Analyze Ventricular Dyssyncrony in SPECT Images
EURASIP Journal on Advances in Signal Processing volume 2010, Article number: 290695 (2009)
Abstract
A method to compute three-dimension (3D) left ventricle (LV) motion and its color coded visualization scheme for the qualitative analysis in SPECT images is proposed. It is used to investigate some aspects of Cardiac Resynchronization Therapy (CRT). The method was applied to 3D gated-SPECT images sets from normal subjects and patients with severe Idiopathic Heart Failure, before and after CRT. Color coded visualization maps representing the LV regional motion showed significant difference between patients and normal subjects. Moreover, they indicated a difference between the two groups. Numerical results of regional mean values representing the intensity and direction of movement in radial direction are presented. A difference of one order of magnitude in the intensity of the movement on patients in relation to the normal subjects was observed. Quantitative and qualitative parameters gave good indications of potential application of the technique to diagnosis and follow up of patients submitted to CRT.
1. Introduction
The automatic quantification of dynamic events, like the heart movement, is one of the most challenging applications in the field of medical image analysis. The normal Left Ventricle (LV) wall deformation occurring throughout the cardiac cycle may be affected by cardiac diseases. Thus, some pathological conditions could be identified by the change they produce in the expected normal movement [1].
Ventricular dyssyncrony is an example of a condition that modifies the normal behavior of the cardiac muscle [2]. Cardiac Resynchronization Therapy (CRT) is one of the procedures applied to patients with intraventricular dyssynchrony and aims to restore the normal contraction pattern by the stimulation of both right and left ventricles simultaneously [3]. Several studies have shown the effectiveness of CRT in patients with heart failure [4, 5]. However, among the patients submitted to CRT, 25–30% do not respond to the treatment [6–9] (nonresponder). For this reason, when choosing CRT for a patient, several factors have to be considered. Besides being highly complex, it is an expensive therapy [10] and implantation of CRT device is not without risks to the patient [11]. The decision of recommending CRT to a patient is therefore a balance of these risks with its potential benefits.
At present, there is a lack of specific measures to characterize the degree of synchrony [12] as well as a factor, which prior to the application of the CRT, can discriminate patients who are going to respond to the therapy from those who are not. A number of researchers have been working to reach this goal in the last years [12–14]. Recently, several studies have used gated scintigraphic images to evaluate the ventricles synchrony by means of phase and amplitude images [10]. However, these two techniques involve a global analysis and may cause a loss of important information about the regional movement of the walls.
Electrocardiographic gating of Cardiac Single-Photon-Emission Computed Tomography (gated-SPECT) provides the clinician with a temporal set of 3D images that enables the visualization of the distribution of radioactive counts within the myocardium and surrounding structures throughout the cardiac cycle. It provides the ability to determine the severity of abnormalities in wall motion and wall thickening associated with myocardial dysfunction [15]. A number of techniques have been used in order to describe and quantify the nonrigid motion of the cardiac structures. Among these techniques, Optical Flow methods are used to accurately model nonrigid motion present during the cardiac cycle so that a one-to-one mapping is found between each voxel of two gated volumes [16, 17].
In previous works, we have described cardiac motion by means of the velocity flow field. The velocity estimation for each voxel in a volume was based on Optical Flow techniques [16]. In this technique, 3D LV motion is described by a series of 3D velocity vector fields computed automatically for each voxel on the sequence of cardiac volumes. The analysis and even the visualization of the velocity field in a cardiac volume are extremely difficult tasks, due to the high amount of information presented simultaneously. To make this bunch of information useful for diagnostic purposes, it is necessary to find compact and friendly representations for it.
In this work we propose a color coded visualization scheme for the qualitative analysis of the velocity components, with the definition of three movement directions. The coded velocity information obtained from Optical Flow in SPECT images is used to assess some aspects of CRT. In particular, we investigate the ability of velocity derived measurements to assess the effectiveness of CRT and velocity patterns that might be able to distinguish responder patients from the nonresponder, before the application of CRT. The assessment is performed on sets of images from thirty normal subjects and sixteen patients with idiopathic dilated cardiomyopathy.
2. Material and Methods
In this section the proposed methods to compute (Section 2.1) and analyze (Section 2.2) the left ventricle motion are described. In Section 2.3 the image acquisition protocol and data sets used for methods evaluation are presented, as well as the criteria used for classification of the patients as responders or nonresponders to the CRT.
2.1. Description of Heart Movement Through Velocity Fields
2.1.1. Velocity Field Calculation
The velocity fields are obtained by using an extension to 3D of the classical 2D Optical Flow [16, 17]. In this approach, two assumptions are imposed to the model. The first is a brightness constancy assumption and it assumes that the intensity of image elements is conserved between the image frames (called the OF constraint). The second assumption consists of a "smoothness" constraint and imposes that in a neighborhood the voxels have similar velocities. The two assumptions are combined in a weighted function as follows:

where the first term is the OF constraint, the second is a measure of the Optical Flow field smoothness, and  is a weighting factor that controls the influence of the smoothness constraint. Ex, Ey, Ez and Et are the image derivatives in the x, y, z and t directions; u, v and w are the components of the local velocity vector v along the x, y and z directions, respectively.
is a weighting factor that controls the influence of the smoothness constraint. Ex, Ey, Ez and Et are the image derivatives in the x, y, z and t directions; u, v and w are the components of the local velocity vector v along the x, y and z directions, respectively.
The minimization of this function leads to a linear algebraic system, whose solution is the velocity component to each voxel and the coefficients are determined by the spatial and temporal derivatives of the images as follows:

where  ,
,  , and
, and  are the mean velocities in each direction, for the voxels in a neighborhood of a given voxel, and n is the iteration index.
are the mean velocities in each direction, for the voxels in a neighborhood of a given voxel, and n is the iteration index.
2.1.2. Computational Description of the Left Ventricular Movement
Generally speaking, the heart can be described as a nonrigid object that deforms throughout the cardiac cycle and has very complex mechanical properties [16]. To simplify the analysis of the left ventricular movement, it can be described in terms of contraction/expansion and torsion. In order to qualitatively evaluate the movement of the LV, three movement directions were defined, each with two possible orientations. These directions are depicted in Figure 1. Radial movement is described as a contraction towards the center of the LV during systole and as an expansion from the center during diastole. Horizontal rotation represents the clockwise and counterclockwise movement of the cardiac walls and the vertical rotation represents the movement towards the base (upwards) during systole and towards the apex during diastole. For the apex, only the radial component, which is the major component of its movement, is analyzed.
Motion directions defined in the cardiac cycle: (a) radial movement in terms of contraction and expansion; (b) horizontal rotation is either clockwise or counterclockwise; and (c) vertical rotation is upwards or downwards. The two orientations for each direction are colored by the defined coding scheme (see Section 2.1.1). Movements are depicted using the short axis view in (a) and (b) and the horizontal long axis view in (c). The LV walls are depicted in (a) left: region 1 is the anterior wall; region 2 is the inferior wall; region 3 is the septal wall; and region 4 is the lateral wall. (The nomenclatures of cardiac planes and wall segments used in this work follow the recommendations of the American Heart Association, as described in Cerqueira et al. [18].)
2.2. Qualitative Analysis of the Movement: Color Coding the Velocity Field in Spherical Coordinates
2.2.1. Spherical Coordinate System
The solution to the algebraic linear system presented in (1) gives the values of the velocity components for each voxel of the cardiac volume in Cartesian coordinates. However, the spherical coordinate system is a more suited system for the description of the movements presented in the former item. For this reason, the visualization module first performs a transformation of the velocities obtained in x, y, and z directions to the unit vectors in the directions  in the spherical coordinate system (Figure 2).
in the spherical coordinate system (Figure 2).
The radial movement can be described by the unit vector for the  component, the horizontal rotation by the unit vector for the
component, the horizontal rotation by the unit vector for the  component, and the vertical movement by the unit vector for the
component, and the vertical movement by the unit vector for the  component.
component.
The center of the spherical coordinate system is essential when representing the left ventricular motion, as the origin is the reference point for the motion. The results for the velocity components are going to be highly dependent on the choice of this point. How to choose the center of the left ventricle is not a simple task. The anatomical center or the center of mass might be used as a central point, but this choice would fail to find the center in images from patients with myocardial infarction or any disease in which the counts are decreased at certain regions of the cardiac muscle. In this work, the center is defined as the geometrical center of the LV and is selected manually by a trained physician.
2.2.2. Color Scheme
A desired feature of the visualization scheme is that all information concerning a movement direction be presented in a single image. Thus each image must present information about the orientation and the intensity of the velocity component. The color coding scheme is therefore defined as following: for each component, the color assigned to a voxel indicates the orientation of the movement, being either positive or negative, and the strength of the color indicates the intensity of the velocity vector in this direction.
Positive and negative orientations for each movement direction are defined as follows:
-
(i)
Radial: expansion is positive; contraction is negative,
-
(ii)
Horizontal rotation: clockwise rotation is negative, and counterclockwise rotation is positive;
-
(iii)
Vertical rotation: downwards motion is positive, and upwards rotation is negative.
In order to indicate velocity intensity, a discrete lookup table is used. In this table, the absence of motion is depicted as white, positive values are depicted as blue, and negative values are depicted as red. The positive and negative colors are divided into 128 steps by changing their saturation, such that strong movement is represented by a strong color, and a weaker movement has a lighter color. Figure 3 shows an example of a color scheme, in which the color variation follows a linear scale. Logarithm scales can be used for better visualization of weak movements.
2.3. Acquisition and Processing of Patient Images
The method was applied to 3D gated-SPECT (99mTc-MIBI) images obtained from sixteen patients with idiopathic dilated cardiomyopathy, chronic heart failure in New York Heart Association functional class III or IV, LV Ejection Fraction  35% and left bundle branch block (QRS
35% and left bundle branch block (QRS  120 milliseconds), referred for implantation of a CRT device. The proposed protocol was approved by the Ethics Committee of the University of Sao Paulo Medical School and an informed consent was obtained from all study subjects and/or their families. The image acquisitions were performed at the Nuclear Medicine Department of the Heart Institute (InCor) HCFMUSP. All acquisitions were performed after the intravenous injection of 10 mCi of [technetium-99 m] sestamibi at rest in a dual-head rotating gamma camera (ADAC Cardio-MD with a LEAP Collimator). The acquisition process is synchronized with the electrocardiogram and the cardiac cycle was divided into 8 frames/cycle. A total of 64 projections were obtained over a semicircular 180-deg orbit. All projection images were stored using a
120 milliseconds), referred for implantation of a CRT device. The proposed protocol was approved by the Ethics Committee of the University of Sao Paulo Medical School and an informed consent was obtained from all study subjects and/or their families. The image acquisitions were performed at the Nuclear Medicine Department of the Heart Institute (InCor) HCFMUSP. All acquisitions were performed after the intravenous injection of 10 mCi of [technetium-99 m] sestamibi at rest in a dual-head rotating gamma camera (ADAC Cardio-MD with a LEAP Collimator). The acquisition process is synchronized with the electrocardiogram and the cardiac cycle was divided into 8 frames/cycle. A total of 64 projections were obtained over a semicircular 180-deg orbit. All projection images were stored using a  , 16-bit matrix. The transverse tomograms were reconstructed with a thickness of 1 pixel/slice (6.47 mm). The volume of transverse tomograms was reoriented, and sets of slices perpendicular to the long axis (short axis view) and of slices parallel to the long axis (vertical long axis view and horizontal long axis view) were created. For each patient the images were acquired in two different conditions: at rest and after pharmacological induced stress.
, 16-bit matrix. The transverse tomograms were reconstructed with a thickness of 1 pixel/slice (6.47 mm). The volume of transverse tomograms was reoriented, and sets of slices perpendicular to the long axis (short axis view) and of slices parallel to the long axis (vertical long axis view and horizontal long axis view) were created. For each patient the images were acquired in two different conditions: at rest and after pharmacological induced stress.
From the group of sixteen patients, eight were responders to the CRT (Group1), and eight patients were nonresponders (Group2). For each patient, the rest and stress data sets were analyzed before to and after CRT, respectively. This gave a total of 64 gated-SPECT data sets included in this analysis. Before the implantation of the CRT device, the clinical condition of the patients was assessed and they were subsequently scanned with three different image modalities: gated-SPECT, echocardiography, and gated blood pool imaging. The aim was to gain an estimated left ventricular Ejection Fraction (EF) from each image modality, for later use as a quantitative measure of the response. After a three-month follow-up, the patients were submitted to the same procedures as prior to CRT. The majority of patients improve immediately their EF or functional class post-CRT implant. Estimates of the EF from each image modality were acquired a second time and compared with the estimated baseline EF. A positive response to CRT was defined as an increase of at least 5 percent points in one or more of the three modalities in addition to a positive clinical assessment. Patients who showed a positive response are named responders, and the ones who did not are named nonresponders.
The method was also applied to image sets of thirty normal subjects (The normal subjects whose images were used in this work were part of a Research protocol approved by the Ethics comittee of the University of Sao Paulo Medical School.), whose acquisition protocol is the same as the one described for the patient images.
3. Results: Application of the Method to Investigate Some Aspects of CRT
3.1. Results for Normal Subjects
By analyzing normal left ventricles, the resulting visualization of the motion patterns can be compared with the motion expected from the heart physiology (seen in Figure 1).
As an example, Figure 4 shows the results obtained for one normal subject using the velocity color coding scheme. The Figure depicts the velocity images for the three movement directions in a slice from the midcavity portion at both diastole (line 1) and systole (line 2). Column (a) presents the images of the radial component, column (b) presents the images of the horizontal rotation component, and column (c) presents the images of the vertical component. The color table used in Figure 4 (as in the remaining figures of this text) is adjusted to the maximum value of each map.
Velocity images of a normal subject in a slice from the midcavity portion of the LV. Line 1 presents diastolic images and line 2 presents systolic images. Column (a) depicts the images of the radial component. The several tones of blue in the myocardium in Line 1 represent the expansion while the tones of red in the same region in Line 2 represent the contraction. Column (b) presents the images of the horizontal rotation component. The tones of blue represent counterclockwise rotation while the tones of red represent clockwise rotation. Column (c) presents the images of the vertical component. The tones of blue represent downward motion while the tones of red represent upward motion. The color scale is shown at the top right of the figure.
3.1.1. Radial Movement
During systole, as the left ventricle ejects blood, the myocardium contracts starting at the apex and moving upwards to the base. Simultaneously, the septal and lateral walls move towards the center of the left ventricle. Therefore, the expected result in systole is the contraction which is presented in the Figure 4, line 2, column a. In this image, the contraction movement is represented by different tones of red, indicating the contraction with varying intensities. After ejection the heart enters the diastole, where the overall motion is opposite of the contraction. The expected colors are therefore also the opposite of the ones observed in systole. The results of a normal wall behavior can be observed in Figure 4, line 1, column a, where the expansion movement is depicted as different tones of blue.
3.1.2. Horizontal Rotation
The analysis of this movement is quite complicated. If one studies the anatomy and physiology of the subepicardial and subendocardial myofibres during both systole and diastole, it would be expected that images would show opposite rotations in the outer and inner sides of the myocardium. This could not be seen in any of the slices of any subjects. Instead, it seems like different rotary motions govern at different parts of the myocardium. The results in the midcavity slices form four corners, where opposing corners have movements with the same direction (see Figure 4, lines 1 and 2, column b). This pattern was similar in all normal left ventricles, hence it was assumed as the normal pattern in the horizontal motion. The results obtained show the expected opposite relationship between systole and diastole.
3.1.3. Vertical Rotation
In the ejection phase, the apex is pressed upwards during contraction to force the blood out through the aortic valve. The expected result of the vertical rotation in systole is therefore an upwards rotation, which is coded as red, this is also seen in Figure 4, line 2, column c. In diastole, the images show movement in the opposite direction.
3.2. Results for Patients
3.2.1. Radial Movement
In both groups of patients, the dyssynchrony of the movement can be observed in the radial movement. After CRT, Group1 should achieve an improved synchronization in the radial motion. This can be seen in most of the analyzed patients, where improvement in synchrony between septal and lateral wall is detected in both systole and diastole. Figure 5 shows an example of such a patient.
Radial motion of a Group1 patient. A slice from the midcavity is shown in systole and diastole, before and after CRT. In systole before CRT, the arrows indicate the septal and lateral walls. It can be noticed that in the septal wall, pixels present positive values—blue color—while in the lateral wall they present negative values—red color. The expected normal movement would be an overall contraction of the walls, represented by red color. Such movement can be seen in systole after CRT. The arrows in the figure indicate the global contraction movement depicted in tones of reds. The color scale is shown at the top right of the figure.
Prior to CRT a dyssynchrony is present in form of a blue (expanding) septal wall and a red (contracting) lateral wall. In the image after CRT, an improved synchrony is visible; here the blue color in the circle is replaced by a weak red color. During diastole in Figure 5, only the lateral wall is expanding before CRT, as the septal wall was expanding in systole. After CRT a more synchronic expansion is detected. It is seen that the overall intensity of the movement is weaker when compared to the normal subjects. The analysis of synchrony in the Group2 showed that there was no improvement in most of patients, as expected.
3.2.2. Horizontal Rotation
The images obtained before CRT present patterns quite different from the one assumed as the normal pattern. However, most of the results for Group1 after CRT are like the pattern found for the normal left ventricles; an example is shown in Figure 6. The example in Figure 6 further shows that the desired opposite relationship between systole and diastole is present.
For Group2 no such pattern in the horizontal rotation was detected. One patient showed a pattern similar to a normal pattern in systole, but a worsening in diastole, while others showed the opposite or a mixture of rotations. None of the patients had a similar pattern of improvement or deterioration in synchrony. The intensity of motion was similar prior to and after CRT in all patients in systole, but in diastole half of the patients had a high intensity in horizontal rotation before CRT, which decreased after CRT. This behavior of noticeable decreased velocity intensity values was not detected in the Group1.
3.2.3. Vertical Rotation
A dyssynchrony in the vertical rotation is present in varying degrees in the results, which is expected for heart failure patients. Most of Group1 patients showed an improvement after CRT. For Group2, however, the normal pattern was hardly obtained even after CRT. In the case of horizontal rotation mentioned in the former section, some Group2 patients had a high intensity in diastole. The same patients had a high intensity in the vertical rotation in diastole. As it was seen in the horizontal rotation, the intensity of the vertical rotation also decreased after CRT. Figure 7 presents the results for a Group1 patient
3.3. Analysis of Radial Movement—Normal and Patient
Table 1 presents the normalized mean velocity values for the radial motion of the anterior, inferior, septal, and lateral walls (Figure 1(a)) for the LV midcavity portion. It presents the values found for one normal subject and one patient of each group (responder and nonresponder).
 and a correspondent minimum of
and a correspondent minimum of  .
.The comparison between patients (Group1 and Group2) and normal subjects shows that not only the synchrony of the movement is compromised, but the intensity is seriously decreased in this set of patients, which reflects the impaired heart function. The numerical values representing the quantity of movement of the normal subjects are ten times higher than the patients. Although this quantity does not change considerably before and after the CRT, the responder patient presents an overall increase in the clinical conditions due to the fact that the synchrony of the movement has been restored. This fact can be seen in Table 1 by the change in the expected sign for the measurement. Patients from the Group2 did not present this improvement in synchrony.
4. Discussion and Conclusions
The analysis of the velocity field from cardiac volumes can give important clues about the dynamic events occurring during the cardiac cycle, which may help to understand how some treatments improve heart function. In this work, the results were presented in a slice of the short axis view and we proposed a scheme for displaying the wall movements which are displayed using a compressive color code that integrates orientation and intensity of the velocity vector at each voxel.
The most important feature of this method is its capability to evaluate LV motion in a more comprehensive way since it allows a regional analysis by assessing the movement in three predefined directions. Other techniques (like echocardiography and phase images derived from Fourier transform of radionuclide ventriculography or even gated single photon emission computed tomography) use previously defined points (or regions) and establish a comparison between them or evaluate indices that characterize global LV synchrony [2, 7, 10, 19].
In this study, the results from the normal subjects were used as the reference for normality in each of the directions. The representation of the velocity components in a color coded image has shown to be an efficient tool for regional inspection of the LV wall movement that could improve the optimal site of LV electrode implant. Actually, the method allows a local analysis, since the results are obtained for each voxel of the volume. This is an important advantage of this method when compared to other global techniques such as phase and amplitude.
Table 1 shows a quantitative comparison of one data set obtained in normal controls, and two patients, one who responded to CRT and one nonresponder. A difference of one order of magnitude in the intensity of the movement on patients in relation to the normal subjects was observed. The evaluation of radial motion before CRT in a nonresponder patient (group 2) showed a movement pattern different from normal in both phases of the cardiac cycle. The responder (group 1) showed motion in the opposite direction from normal controls only in inferior and septal walls. This fact could suggest that responders are different from nonresponders before therapy. After therapy, the direction of the motion of inferior and lateral walls of the nonresponder became similar to normal controls, but not the direction of anterior and septal walls. The group 1 patient showed a normal motion pattern except in inferior wall after therapy. The qualitative and quantitative parameters obtained with this method could add information to a better selection of patients who would respond to TRC and provide a measurable tool to the follow-up in this population.
Limitations
the spherical coordinate system was chosen for calculating the orientation and intensity of the left ventricular motion. A key issue to the proposed scheme is the center of the spherical coordinate system since it is the reference point for the motions and therefore essential in the visualization of the velocity components. A change in center will influence both the intensity and orientation of the left ventricular motion. Choosing the center is difficult as it should be the exact point or axis from which the motion starts and ends. In the present work, the center was determined manually by a trained observer as the geometrical center of the LV.
Another limitation is the poor resolution of SPECT images that sometimes makes it difficult to analyze the movements. It must be added, however, that the proposed method is not applicable to nuclear medicine imaging only and can be extended to other modalities.
Future Perspectives and Conclusions
the results are preliminary indications obtained via a qualitative assessment. Quantitative indexes can be created based on these images that would be able to quantitatively assess both the effectiveness and prediction of CRT response. These indexes could be based on the creation of normal distributions of the velocity field for each direction. An alternative and elegant approach for defining quantitative tools for the analysis of the movement patterns is the creation of a functional bull's eye [20–23]. Once the bull's eyes of the described movement patterns have been built, many studies can be performed for the assessment of the patient's condition. In order to find an index to predict response to CRT therapy, extensive clinical studies must be performed and involve the acquisition of a statistically significant number of images from normal subjects and patients.
In this study, the left ventricular three-wall movements were studied using a compressive color code that characterizes the integration of orientation and intensity of the velocity vector at each voxel. This new technique of myocardial synchronization assessment might be able to distinguish responder patients from the nonresponders and improve the follow up of patients who underwent CRT.
References
Remme EW, Young AA, Augenstein KF, Cowan B, Hunter PJ: Extraction and quantification of left ventricular deformation modes. IEEE Transactions on Biomedical Engineering 2004, 51(11):1923-1931. 10.1109/TBME.2004.834283
Chen J, Garcia EV, Folks RD, et al.: Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. Journal of Nuclear Cardiology 2005, 12(6):687-695. 10.1016/j.nuclcard.2005.06.088
Rioual K, Unanua E, Laguitton S, et al.: MSCT labelling for pre-operative planning in cardiac resynchronization therapy. Computerized Medical Imaging and Graphics 2005, 29(6):431-439.
Auricchio A, Abraham WT: Cardiac resynchronization therapy: current state of the art: cost versus benefit. Circulation 2004, 109(3):300-307. 10.1161/01.CIR.0000115583.20268.E1
Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert J-C: Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the MUltisite STimulation In Cardiomyopathy Study (MUSTIC). American Journal of Cardiology 2003, 91(9):1090-1095. 10.1016/S0002-9149(03)00154-1
Lecoq G, Leclercq C, Leray E, et al.: Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. European Heart Journal 2005, 26(11):1094-1100. 10.1093/eurheartj/ehi146
Bleeker GB, Bax JJ, Fung JW-H, et al.: Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. American Journal of Cardiology 2006, 97(2):260-263. 10.1016/j.amjcard.2005.08.030
Yu C-M, Bleeker GB, Fung JW-H, et al.: Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005, 112(11):1580-1586. 10.1161/CIRCULATIONAHA.105.538272
Yeim S, Bordachar P, Reuter S, et al.: Predictors of a positive response to biventricular pacing in patients with severe heart failure and ventricular conduction delay. Pacing and Clinical Electrophysiology 2007, 30(8):970-975. 10.1111/j.1540-8159.2007.00794.x
Brandão SCS, Giorgi MCP, de Miche RT, et al.: Ventricular synchrony in patients with dilated cardiomyopathy and normal individuals: assessment by radionuclide ventriculography. Arquivos Brasileiros de Cardiologia 2007, 88(5):596-601. 10.1590/S0066-782X2007000500016
Strickberger SA, Conti J, Daoud EG, et al.: Patient selection for cardiac resynchronization therapy: from the Council on Clinical Cardiology Subcommittee on Electrocardiography and Arrhythmias and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation 2005, 111(16):2146-2150. 10.1161/01.CIR.0000161276.09685.4A
O'Connell JW, Schreck C, Moles M, et al.: A unique method by which to quantitate synchrony with equilibrium radionuclide angiography. Journal of Nuclear Cardiology 2005, 12(4):441-450. 10.1016/j.nuclcard.2005.05.006
Declerck J, Feldmar J, Ayache N: Definition of a four-dimensional continuous planispheric transformation for the tracking and the analysis of left-ventricle motion. Medical Image Analysis 1998, 2(2):197-213. 10.1016/S1361-8415(98)80011-X
Tecelão SRR, Zwanenburg JJM, Kuijer JPA, et al.: Quantitative comparison of 2D and 3D circumferential strain using MRI tagging in normal and LBBB hearts. Magnetic Resonance in Medicine 2007, 57(3):485-493. 10.1002/mrm.21142
Klein GJ, Reutter BW, Huesman RH: Non-rigid summing of gated PET via optical flow. IEEE Transactions on Nuclear Science 1997, 44(4):1509-1512. 10.1109/23.632704
Gutierrez MA, Rebelo MS, Furuie SS, Meneghetti JC: Automatic quantification of three-dimensional kinetic energy in gated myocardial perfusion single-photon-emission computerized tomography improved by a multiresolution technique. Journal of Electronic Imaging 2003, 12(1):118-124. 10.1117/1.1526104
Horn BKP, Schunck BG: Determining optical flow. Artificial Intelligence 1981, 17(1–3):185-203.
Cerqueira MD, Weissman NJ, Dilsizian V, et al.: Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105(4):539-542. 10.1161/hc0402.102975
Brandão SCS, Nishioka SAD, Giorgi MCP, et al.:Cardiac resynchronization therapy evaluated by myocardial scintigraphy with
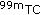 -MIBI: changes in left ventricular uptake, dyssynchrony, and function. European Journal of Nuclear Medicine and Molecular Imaging 2009, 36(6):986-996. 10.1007/s00259-008-1029-1
-MIBI: changes in left ventricular uptake, dyssynchrony, and function. European Journal of Nuclear Medicine and Molecular Imaging 2009, 36(6):986-996. 10.1007/s00259-008-1029-1Qureshi RJ, Husain SA: Design of na expert system for diagnosis of coronary artery disease using myocardial perfusion imaging. Proceedings of the National Conference on Emerging Technologies, 2004 100-105.
Rougon N, Petitjean C, Prêteux F, Cluzel P, Grenier P: A non-rigid registration approach for quantifying myocardial contraction in tagged MRI using generalized information measures. Medical Image Analysis 2005, 9(4):353-375. 10.1016/j.media.2005.01.005
Gérard O, Billon AC, Rouet J-M, Jacob M, Fradkin M, Allouche C: Efficient model-based quantification of left ventricular function in 3-D echocardiography. IEEE Transactions on Medical Imaging 2002, 21(9):1059-1068. 10.1109/TMI.2002.804435
Lin J-W, Laine AF, Bergmann SR: Improving PET-based physiological quantification through methods of wavelet denoising. IEEE Transactions on Biomedical Engineering 2001, 48(2):202-212. 10.1109/10.909641
Acknowledgments
The authors would like to thank Dr. Ramon Moreno, Maurício Higa, and Carlos Santos for their valuable discussions at the elaboration of this work. This work was supported in part by the Foundation of Aid for Research of São Paulo State (FAPESP) Grant no. 2006/06612-4, the National Council for Scientific and Technological Development (CNPq) Grant no. 300499/2005-1, the National Institute of Science and Technology—Medicine Assisted by Scientific Computing INCT MACC, and the Zerbini Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rebelo, M.d.S., Aarre, A.K.H., Clemmesen, KL. et al. Determination of Three-Dimensional Left Ventricle Motion to Analyze Ventricular Dyssyncrony in SPECT Images. EURASIP J. Adv. Signal Process. 2010, 290695 (2009). https://doi.org/10.1155/2010/290695
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1155/2010/290695







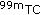 -MIBI: changes in left ventricular uptake, dyssynchrony, and function. European Journal of Nuclear Medicine and Molecular Imaging 2009, 36(6):986-996. 10.1007/s00259-008-1029-1
-MIBI: changes in left ventricular uptake, dyssynchrony, and function. European Journal of Nuclear Medicine and Molecular Imaging 2009, 36(6):986-996. 10.1007/s00259-008-1029-1